Psoriasis support : eHealth gaming tools for patient engagement
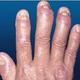
| Psoriasis manum (Photo credit: Wikipedia) |
Here is the IFPA survey to compare 17 different strategies and activities that can be used to advance psoriasis education, advocacy and awareness. Preliminary results of the survey will be presented on World Psoriasis Day and the final results will be announced at the 4th World Psoriasis & Psoriatic Arthritis Conference in Stockholm July 8-11, 2015.
I have listed below some of my random ideas on eHealth tools for patient engagement in psoriasis:
An Agent based model (ABM) offers visual simulations of complex systems that can be displayed on a web browser. Psoriasis disease process can be modelled using psoriasis patients as ‘turtles’ with the known probabilities of auto-remission, exacerbation, response to conventional treatments and response to newer drugs added to the model. The patients and caregivers could interact with the model to understand how the treatment decisions affect the quality of life. ABM could be an innovative and useful web based patient education tool that portrays the reality of psoriasis without giving any false promises. Those in the patient’s circle of care and the patient would understand the odds of improving quality of life.
| Psoriasis: The naked truth (Photo credit: SomosMedicina) |
An android or iPhone app to calculate and log the PASI score of the patient would be a less obtrusive disease monitoring tool. The app may be designed to send the log to patient’s caregiver. I have not checked the apple app store or google play, probably such apps already exist.
A ‘push’ strategy such as email alerts is unlikely to work for psoriatics. An innovative strategy game where the body is modelled as a kingdom and the immunological perturbations as a t-cell mutiny could be a useful engagement tool. Vascular and systemic changes could also be part of the game. The game would be web based and would continue for a long time with the patient required to login periodically to make strategic alterations (treatment choices). Everytime the patient login to the game, medication reminders would be displayed. The game would mimic reality with changes reflecting new clinical studies. New clinical studies that has an impact on the ‘game plan’ would be available under the ‘game resources’ for everyone to read. Reading and understanding these resources would improve the performance in the game.