The great anti-ageing mantra
Matrix metalloproteinases (MMPs) are important in dermatology as they are involved in remodelling extracellular matrix (ECM). It is a big family of enzymes, all having a common domain structure with a pro-peptide region, catalytic domain, hinge region and the haemopexin-like C-terminal domain. They are involved in a variety of biological functions. MMP1 is involved in the premature ageing in smokers.[1] Inhibition of certain MMPs have anti-ageing effect while inhibition of others promote hyperproliferative disorders and scars. However because of the structural similarities, rational design of specific inhibitors are very difficult. Most of the rationally designed MMP inhibitors like marimastat (BB-2516) and cipemastat (Ro 32-3555) have performed poorly in clinical studies.
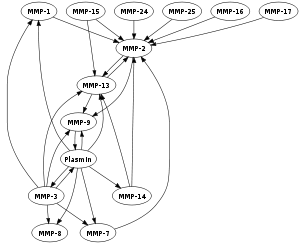
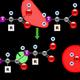
| This is a Graph showing the mutual activation of matrix metallo-proteinases. (Photo credit: Wikipedia) |
This paper [2] proposes a new approach wherein regulatory sites hidden and scattered over different locations on the protein’s surface are targeted instead of the catalytic site using specially designed branched amphiphilic molecules. They have demonstrated that MMP family possess a unique set of such sites making it possible to target specific MMPs without affecting others. If this technology can be successfully developed it may have several applications including many dermatological uses.
References:
1. Lahmann, Christine et al. “Matrix metalloproteinase-1 and skin ageing in smokers.” The Lancet 357.9260 (2001): 935-936.
2. Udi, Yael et al. “Unraveling hidden regulatory sites in structurally homologous metalloproteases.” Journal of Molecular Biology (2013).